Backlinks
1 Coulomb's Law
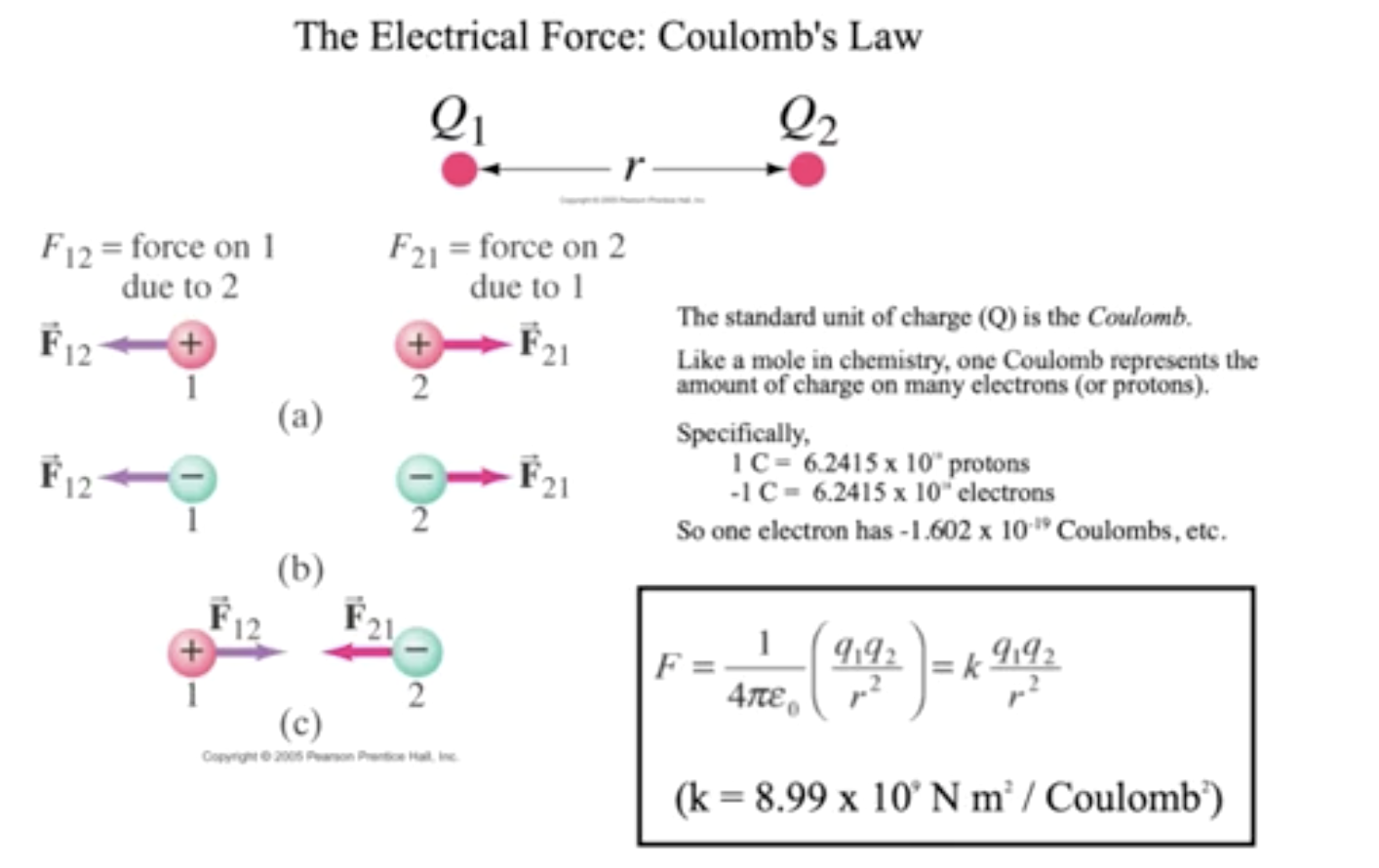
Figure 1: Screen Shot 2020-08-24 at 7.40.48 PM.png
- Electrical forces gets stronger as charge increases
- Electrical forces gets weaker as charge decreases
The magnitude of force that a particle, \(q1\), has upon another \(q2\), is given by the Coulumb's law
\definition[where $k$, a constant for change, $q_1$, change of first particle, $q_2$, change of second partical, $r^2$, distance squared]{Coulumb's Law}{\(k\frac{q_1q_2}{r^2}\)} Note! The Standard Unit of Charge (Q) is the Coulomb — a representation for change for many electrons or many protons
Remember this!
\definition{Charge of an Electron}{\(-1.602 \times 10^{-19} Q\)} \definition{k}{\(8.99 \times 10^9 \frac{Nm^2}{Q^2}\)}
E.M. forces, really, are two forces interacting with each other
Notice! Be careful with the signs when applying coulumbs law
- If resulting Coulomb force > 0, force is REPULSIVE (became you multiplied positive to positive or negative negative)
- If resulting Coulomb force < 0, force is ATTRACTIVE (became you multiplied positive to negative)
Coloumb's law could be applied when modeling KBhPHYS201ElectricFields Electric fields to see how particles interact and how they influence each other.
1.1 Guided Problem Solve
Special care must be taken for solving these problems w.r.t. to both vector direction and multiple-atom-interactions KBhPHYS201GuidedProblemCoulomb
1.2 Here's something! DNA Replication
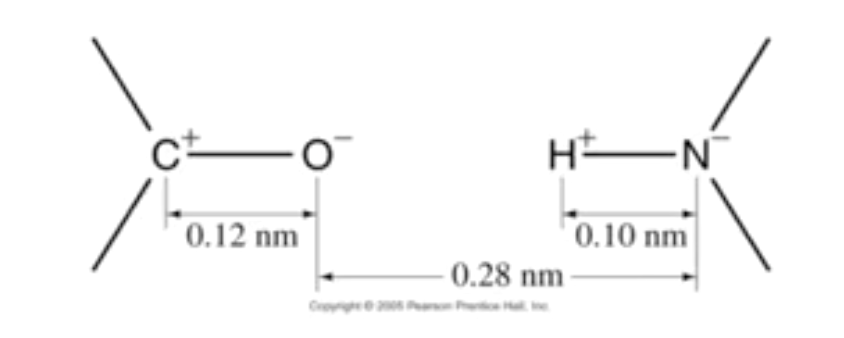
Figure 2: Screen Shot 2020-08-24 at 8.20.15 PM.png
The question is… Between these four atoms, how many do we need to calculate to find if these two repel or attract?
This is fairly simple. Because of the fact every force between each pair of atoms between these two elements needs to be calculated. So… 2 (on the left) times 2 (on the right) = 4.
If these repel, the don't combine. If they attract, of course they do.