1 Structure of Lipids
1.1 Fatty acids
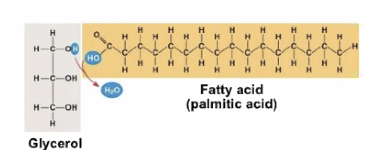
Figure 1: Screen Shot 2020-09-09 at 2.58.49 PM.png
A single penteine and embellishments. Single Fatty acids = Glycerol
1.2 Trygricerol
Fat! (a.k.a. adapose tissue) = Triglycerol: three fatty acids together.
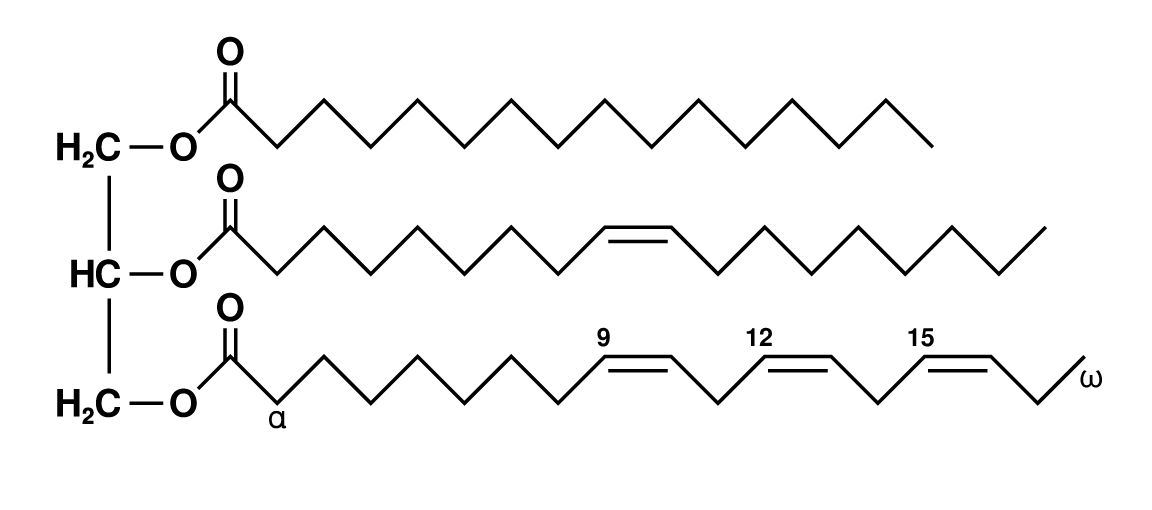
Figure 2: Fattriglycerideshorthandformula.png
1.2.1 Saturated vs. Unsaturated fats
Saturate Fats No double bonds in the carbon chain — think! butter
Unsaturated Fats Double bonds in the carbon chain — think! olive oils
Saturated fats has a higher melting point then the unsaturated fats, but unsaturated fats have double bonds whereas saturated fats have single bonds only. Why?
- Double bonds, due to their caused VESPR geometry (and hence the -1 hydrogen), are curved. This makes it harder to stack together, causing a lower melting point
- Single bonds, due to their caused VESPR geometry, is flat. This makes them easier to stack together, causing a higher melting point.
1.3 Phosophilids
2 fatty acids (hydrophobic) + phosphate group (hydrophillic)
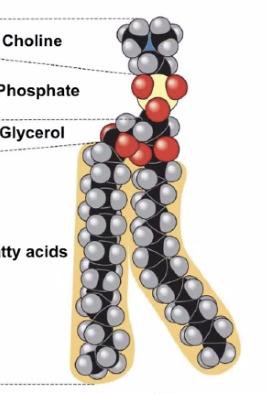
Figure 3: Screen Shot 2020-09-09 at 3.15.41 PM.png
A combination of many of these will end up with membrane:
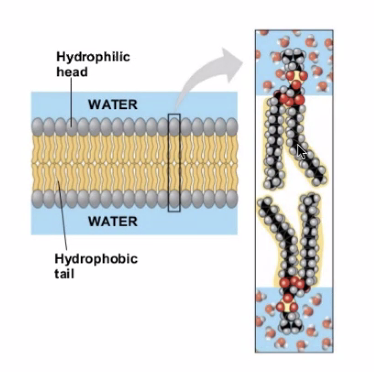
The hydrophobic tail stays inside, and the hydrophillic head pokes outside and attracts water.
1.4 Liposomes + micelles
Lots of phosophillids
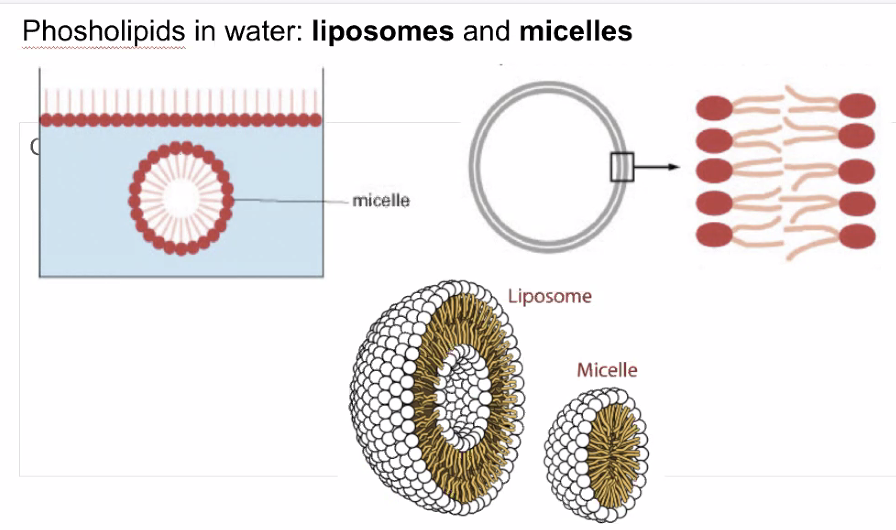
Figure 4: Screen Shot 2020-09-09 at 3.11.54 PM.png
A same idea as Phosophilids, but instead in a big wad of Phosolipids. this arrangement is also how basic cells form membranes. KBhBIO101CellMembraines
1.5 Steroids
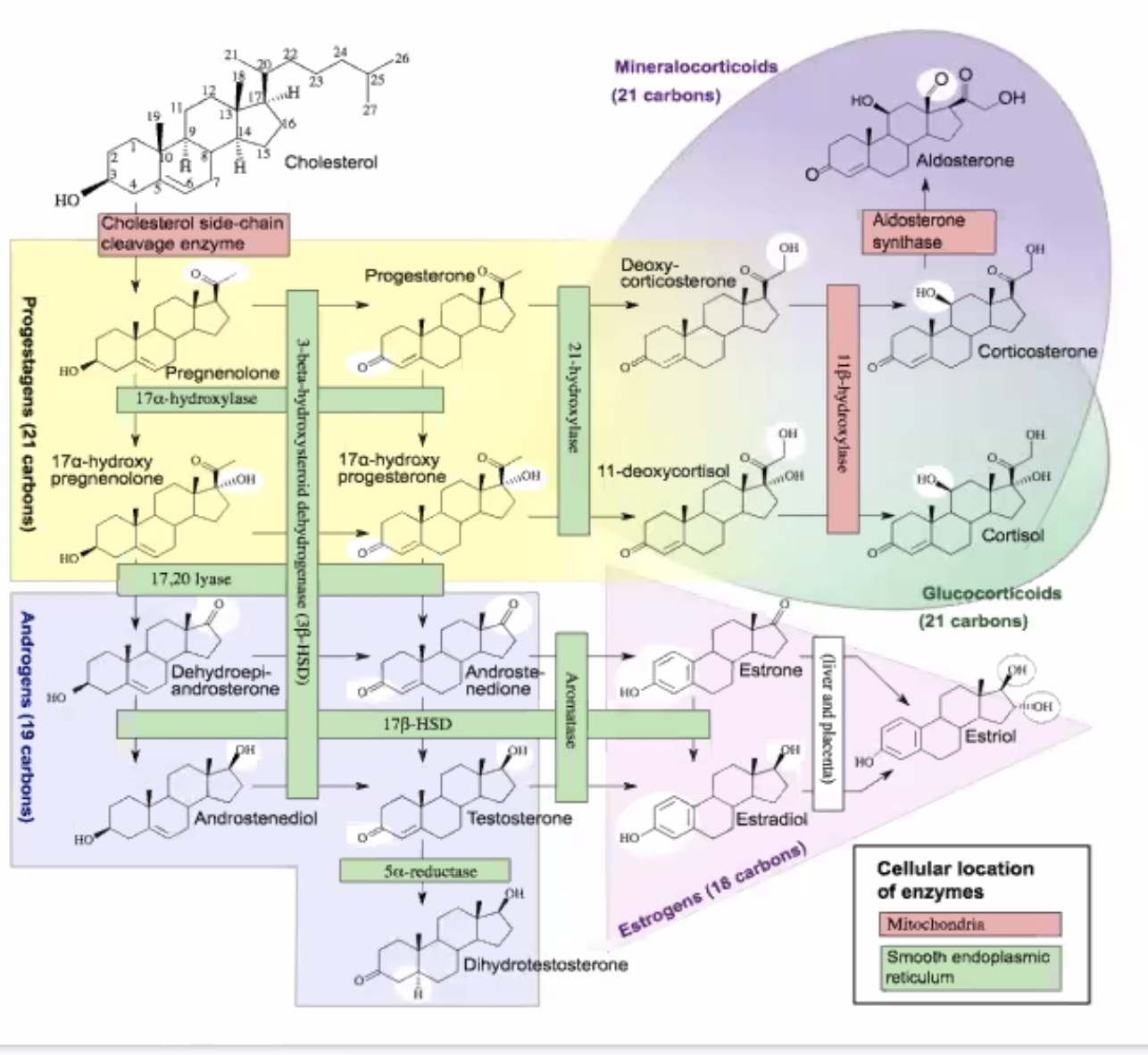
Figure 5: Screen Shot 2020-09-11 at 2.43.35 PM.png
Steroids typically are lipids that contain a ring structure, which usually contains 17 carbon lipids with rings formed by 5-6 carbons each