Backlinks
Table of Contents
1 Lipids
1.1 Pentaine
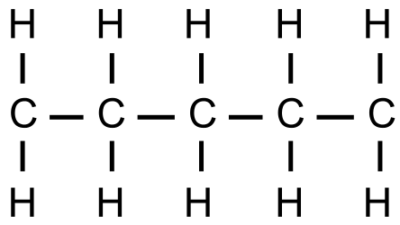
Figure 1: pentane-plus-market-400x225.png
A non-polar molecule that build up the fundamentals of lipids.
- Non polar
- Will only have LDF
- Hydrophobic
- Because it is non polar, will only have LDF
- So, water's dipole moment makes it not attractive to pentaine
- Large surface area of pentaine will result in more LDF (bigger surface area => more opportunities to LDF), so pentaines will more likely to LDF with other pentains if given the choice between pentain vs. water
and now, a note on LDF
Pentaine => Carbon-Carbon bonds has 0 EN difference w/ even distribution of electrons
- Electrons are randomly within their 3D shape
- So, when electrons are accidentally concentrated, a dipole moment will temporarily form
1.2 And now, actually lipids
Lipids are built up from peintaine molecules. You could also take many of these lipids + other elements to build up more complicated, hydrophobic/phillic structures.