1 Alright, let's talk about water.
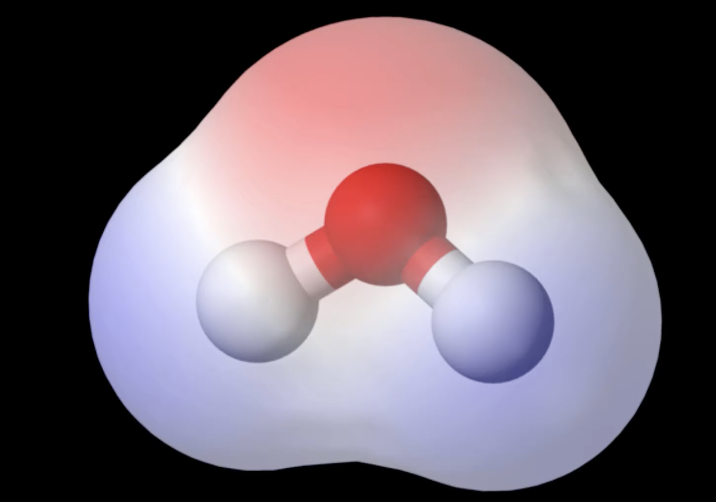
Figure 1: Watah!
1.1 Intra-Molecular water bonds
- As we know (or looked up from the PTable)
- Hydrogen has electronegativity of 2.20, and oxygen has EN 3.44
- The difference >0.4 <1.7 makes these bonds polar covalent
See KBhBIO101BondingReview, bonding review
1.2 Why is ice less dense?
- Freezing usually bring new ordering of molecules to make it paced
- But! Not the case for normal water because of hydrogen bond
- Strong 6-ring Hydrogen bonds in ice prevents shrinking
- Empty space filled with air, making it less dense
1.2.1 Why do I care?
Ice floats! Causing beautiful ice effects that the ecosystem uses
- Pseudo-landmass
- Antarctica
- Animal habitat
- Ice reflect incoming light ("albeto")
- Bounces energy off
- Cools the water
1.3 Inter-Molecular water bonds properties
See KBhBIO101PropsOfWater, properties of water.
2 So, why is water the chosen liquid?
See also KBhBIO101PropsOfWater Properties of Water
- Liquid at Earth temperatures
- Sticky => strong bonds that help water hold together + resist change
in temperature (hence why AlcaholLand cannot exist).
- Varing te (incomplete #todo-houjun @jemoka)
- Is universal solvent