Backlinks
Table of Contents
1 Entropy
#flo #disorganized
Startistical measure of randomness in a reaction of systems.
Entropy measured in microstates — the spead of energy in states. Greater numbers of microstates means that there is more entropy
To think about this, think about states of matter:
- Gas => Most Entropy
- Water => Meh Entropy
- Solids => Least Entropy
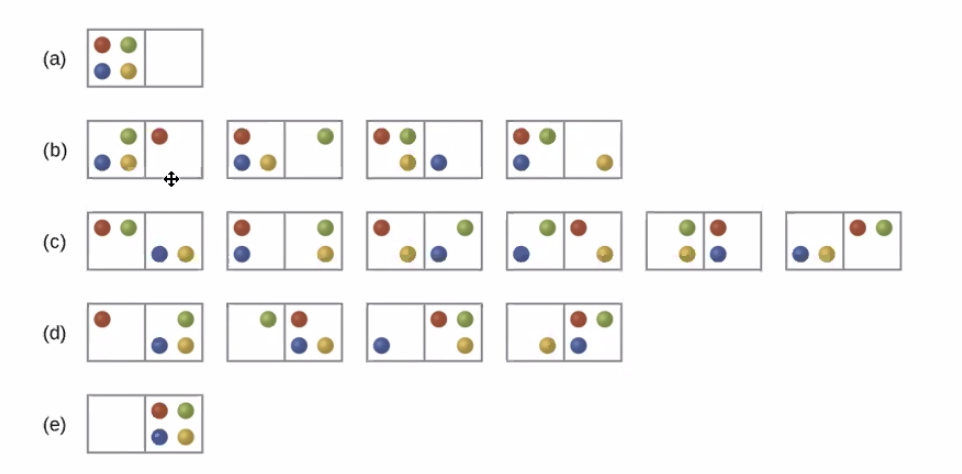
Figure 1: Screen Shot 2020-10-02 at 2.29.24 PM.png
In this image, states (a) and (e) are least likely. This is because *the greater the spread, the greater the entropy; systems like to have an increase of entropic state as much as it is possible.*
\definition{Second Law of Thermodynamics}{In the universe, entropy is increasing due to chemical processes.}
1.1 Gibbs Free Energy
\(\Delta G = \Delta H - t \Delta S\)
Change in gibbs free energy is equal to change in enthalpy minus the change in entropy multiplied by the temperature.
| \(\Delta H\) | \(\Delta S\) | \(-T \Delta S\) | \(\Delta G\) | Spontanety? | Examples? |
|---|---|---|---|---|---|
| + | - | + | + | Non-Favorable Nonspontaneus: creating less entropy, heat is going in. | TBD |
| - | + | - | - | Favorable Spontenous: creating more entropy, heat is flowing out. | Combustion Reactions ( blowing things up) |
| - | - | + | \(\pm\) | Low Temp: Spontaneous High Temp: Nonspontaneus | |
| + | + | - | \(\pm\) | High Temp: Spontaneous Low Temp: Nonspontaneus |